| 4 minute read
Navigating the revised APF26 guidelines for non-sterile expiry dates

The revised guidelines in the Australian Pharmaceutical Formulary and Handbook (APF26) include important changes to the assignment of expiry dates for compounded preparations.
In this article, we explore those changes, the implications for compounding pharmacists, and what to consider when assigning expiry dates.
Background
In compounding, the expiry date is the final date a compounded medication can be used. The in-use expiry date is the amount of time that the medicine can be used after its first opened.
All compounded medicines must be assigned and labeled with an appropriate expiry date and not be in use after the assigned expiry date or in-use expiry date, whichever occurs first.
Factors to consider when assigning an expiry date:
- Route of administration
- Properties of APIs and ingredients
- Dosage form
- Physical and chemical stability
- Potential for microbial contamination and growth, e.g., water-containing medicines, presence of preservatives (and whether they remain effective until the expiry date)
- Final container and container closure
- Storage conditions
The new expiry dates, as outlined in APF26, are as follows:
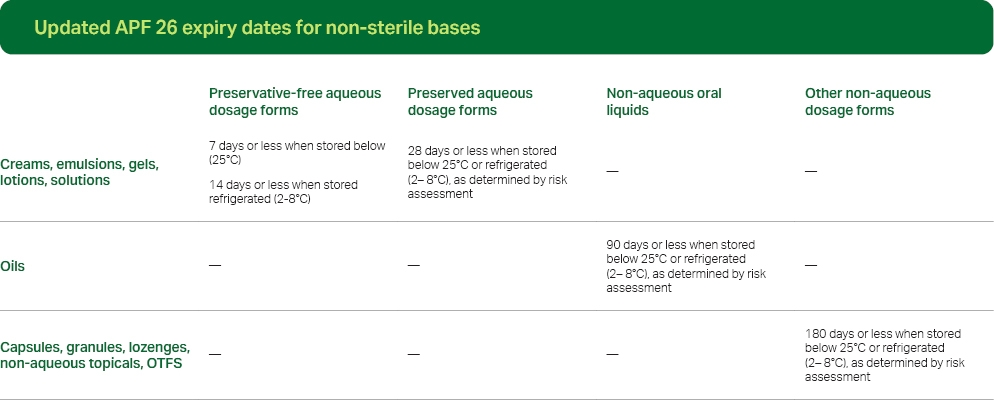 *The above expiry dates are applicable for formulations not specified in a reputable formulary or stability study.
*The above expiry dates are applicable for formulations not specified in a reputable formulary or stability study.The expiry dates of compounded preparations are categorized according to whether the medication is aqueous or non-aqueous (anhydrous). For aqueous preparations, expiry dates are assigned based on preservation. For anhydrous preparations, expiry dates are categorized as either oral liquid or other dosage forms.
Water activity and APF26
Per APF26, water activity (aw) determines whether a dosage form or preparation is classified as aqueous or anhydrous. Water activity is the amount of free, unbound, or available water in a formulation. Different from water content, water activity is the available water to support microbial growth or hydrolytic reactions.
Per current APF26, non-aqueous (or anhydrous) preparations are defined as having aw < 0.6, whereas preparations with aw ≥ 0.6 are considered aqueous.For more information on the impact of water activity in compounded preparations, download our white paper here.
Stability studies
The expiry date of a compounded medicine may be extended beyond the default if a stability-indicating study exists to support the date. The study must be specific to the formulation and uses the same or equivalent method, container, and container closure.
The study must also provide support for physical, chemical, and microbiological stability until the expiration date.
The expiry date of any compounded medication must not exceed 180 days from the date of compounding.
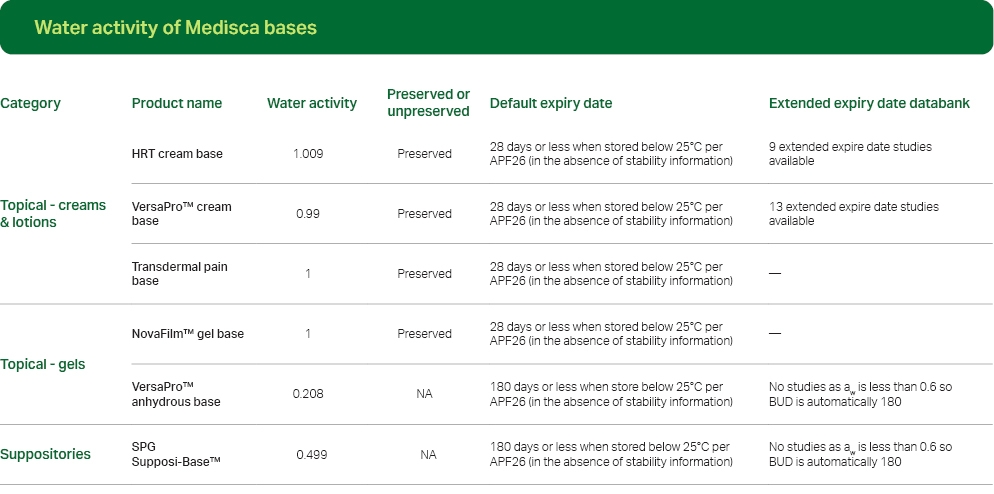
Click here to view all bases and their water activity.
Our extensive BUD and stability studies libraries offer invaluable resources about the extended shelf life and stability of commonly prepared compounded medications. By leveraging these studies, healthcare providers can make informed decisions, optimize medication management, and enhance patient care.
Disclaimer:
Note on unpreserved aqueous dosage forms
Aqueous formulations must contain a suitable antimicrobial preservative system whenever possible (see TGO 100). Preservative-free aqueous formulations must be stored refrigerated (2–8 °C) unless refrigeration changes the formulation properties (e.g., precipitation). If a preservative-free formulation cannot be refrigerated, then it must be supplied in single-dose containers. Preservative-free formulations must be supplied in containers that minimize microbial contamination during use (e.g., pump dispenser, collapsible tube, single-dose oral syringes).Note on preserved aqueous dosage forms
A longer expiry date may be assigned if the pharmacist can document reputable evidence of the physical, chemical, and microbiological stability of the formulation until the expiry date when stored under the specified storage conditions and during administration (e.g., BP or USP–NF antimicrobial effectiveness test). Suspensions and emulsions can be difficult to preserve; antimicrobial preservation should be based on reputable evidence (e.g., BP or USP–NF antimicrobial effectiveness test).
Note on non-aqueous oral liquids
A longer expiry date may be assigned if the pharmacist can document reputable evidence of the physical, chemical, and microbiological stability of the formulation until the expiry date when stored under the specified storage conditions and during administration (e.g., BP or USP–NF antimicrobial effectiveness test).
Note on other non-aqueous dosage forms
Risk assessment must include assessing the physical and chemical stability of the formulation (e.g., hygroscopic properties, incompatibilities).


